
Methyltrichlorosilane (MTCS)
| Chemical Name: | Methyltrichlorosilane(M1 / MTCS) |
| Product Category: | Silicone Monomer – Product |
| Structural Formula: | 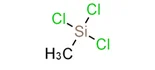 |
| CAS No.: | 75-79-6 |
| EINECS: | 200-902-6 |
| Molecular Formula: | CH3Cl3Si |
| Molecular Weight: | 149.47 |
Methyltrichlorosilane (MTCS) Description
Methyltrichlorosilane (CAS 75-79-6) is a colorless, transparent liquid with a boiling point of 66°C. It is toxic, flammable, and combustible, requiring careful handling.
This silane compound is a key raw material in the silicone industry, primarily used to produce methyltriethoxysilane, methyltrimethoxysilane, and other crosslinking agents. It is also widely applied in silicone resins, specialty coatings, construction waterproofing agents, and as an anticollapse agent (sodium methylsilicate) in oilfield drilling.
| Flash Point | -9℃ (closed cup) |
| Melting point | -90°C |
| Boiling point | 66℃ |
| Autoignition temperature | 490℃ |
| Relative density (water=1) | 1.28 |
| Relative vapor density (air=1) | 5.2 |
| Vapor pressure | 17.9kPa (20℃) |
| PH value | Reacts with water to form HC. |
| Upper explosion limit [% (V/V)] | 7.6 |
| Lower explosion limit [%”/7)] | 20 |
| Water solubility | reacts with water |
| Appearance | colorless transparent liquid |
| Methyltrichlorosilane mass fraction / % | ≥99.0 |
| Mass fraction of trichlorotrimethylsilane / % | ≤0.1 |
| Silicon tetrachloride mass fraction / % | ≤0.1 |
| Hazardous |
|
| Transportation & Safety info |
|
| Packaging |
|
| Sample |
|
| Inventory items |
|
| Price |
|
Packaging Specifications


Jessica G.
Get the Latest Pricing and Information
- Quick and helpful reply within 8 hours;
- Tailored solutions provided for your project;
- One-stop purchasing service.
Methyltrichlorosilane (MTCS): Guide
Methyltrichlorosilane (CAS 75-79-6) is a key raw material for producing various organosilicon compounds, including water repellents, fumed silica, methyl silicone resins, and polysiloxanes. It is highly volatile at room temperature and reacts with water to form hydrochloric acid and a white powdery substance. When heated, it decomposes, releasing hydrogen chloride.
This compound is toxic by inhalation, and both its vapor and liquid can cause skin burns. It reacts with anhydrous ethanol to produce methyltriethoxysilane, making it essential for silicone-based applications. Proper handling and storage are crucial due to its reactive nature.












